Syllabus Objectives:s
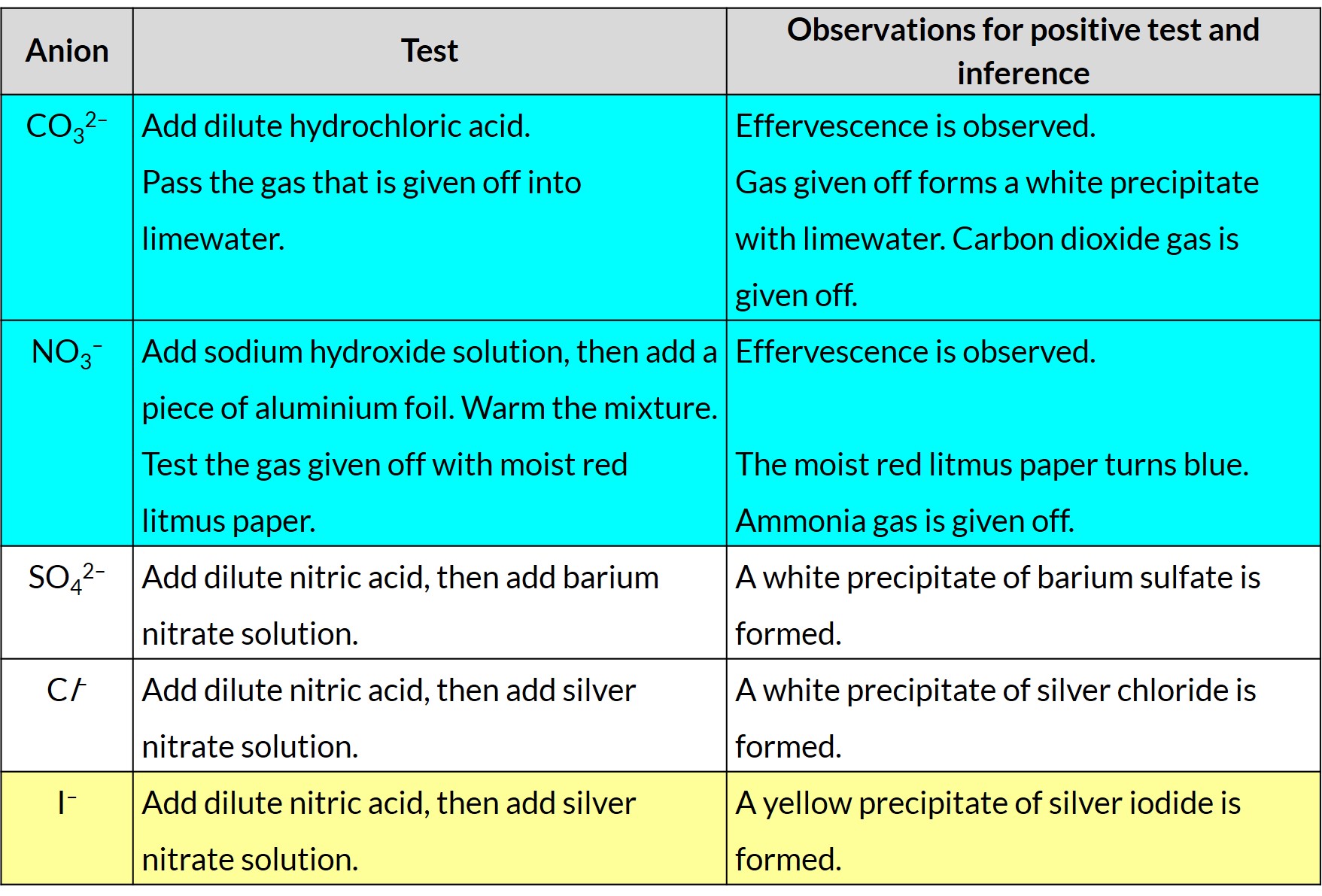
(i) carbonate (by the addition of dilute acid and subsequent use of limewater)
(ii) chloride (by reaction of an aqueous solution with nitric acid and aqueous silver nitrate)
(iii) iodide (by reaction of an aqueous solution with nitric acid and aqueous silver nitrate)
(iv) nitrate (by reduction with aluminium in aqueous sodium hydroxide to ammonia and subsequent use of moist red litmus paper)
(v) sulfate (by reaction of an aqueous solution with nitric acid and aqueous barium nitrate)
Question
Other than barium nitrate solution, what other solution could be added to test for sulfate ions?
Hint: Recall solubility table
Lead(II) nitrate solution. White precipitate of lead(II) sulfate is formed.
Pb(NO3)2 (aq) + Na2SO4 (aq) → PbSO4 (s)+ 2 NaNO3 (aq)
Question
Other than silver nitrate solution, what other solution could be added to test for chloride ions?
Hint: Recall solubility table
Lead(II) nitrate solution. White precipitate of lead(II) chloride is formed.
Pb(NO3)2 (aq) + 2 NaCl (aq) → PbCl2 (s)+ 2 NaNO3 (aq)
Question
Other than silver nitrate solution, what other solution could be added to test for iodide ions?
Lead(II) iodide solution. Yellow precipitate of lead(II) iodide is formed.
Pb(NO3)2 (aq) + 2 NaI (aq) → PbI2 (s)+ 2 NaNO3 (aq)

The lesson was awesome in simple terms
LikeLike