Redox Basics
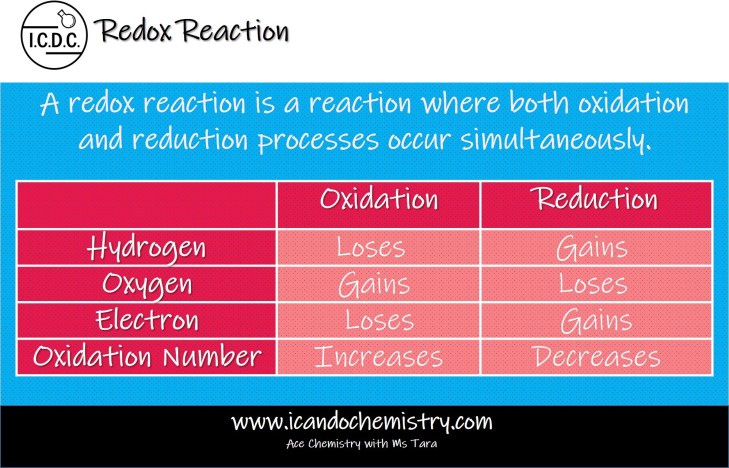
A redox reaction is a reaction where both oxidation and reduction processes occur simultaneously.
Oxidation and reduction usually take place together.
Oxidation occurs when there is a gain of oxygen, loss of hydrogen and loss of electrons.
Reduction occurs when there is a loss of oxygen, gain of hydrogen and gain of electrons.
Oxidation state increases when oxidation occurs.
Oxidation state decreases when reduction occurs.
Oxidising and Reducing agent
Oxidising agent is a chemical substance, which causes another substance to be oxidised while itself is being reduced.
Reducing agent is a chemical substance, which causes another substance to be reduced while itself is being oxidised.
