Tests to identify cations
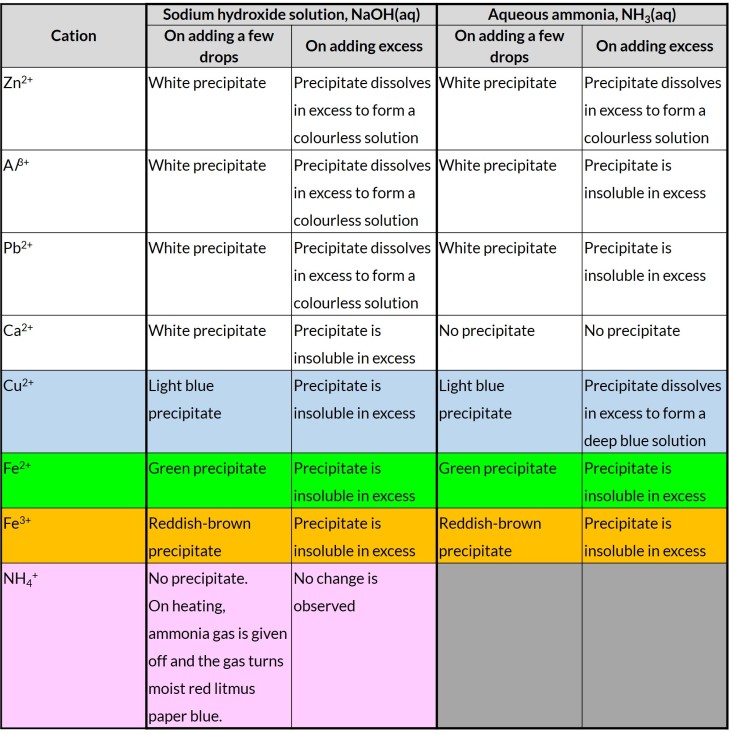
Describe the use of aqueous sodium hydroxide and aqueous ammonia to identify the following aqueous cations: aluminium, ammonium, calcium, copper(II), iron(II), iron(III), lead(II) and zinc (formulae of complex ions are not required).
Reaction with aqueous sodium hydroxide
Outcome 1: No precipitate formed (NH4+)
Ammonium salts will react with aqueous sodium hydroxide to form a soluble salt, water and ammonia gas.
NH4Cl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) + NH3 (g)
On heating, ammonia gas is given off. Gas turns moist red litmus paper blue.
Why should the litmus paper be moist?
Ammonia, being an alkaline gas, produces hydroxide ions (OH–) in the presence of water.
H2O (l) + NH3 (g) → NH4+ (aq) + OH– (aq)
Outcome 2: Precipitate formed (Zn2+, Al2+,Pb2+,Fe2+,Fe3+,Cu2+,Ca2+)
When sodium hydroxide is added to other metal cations, an insoluble hydroxide is formed.
Example: Pb2+
PbCl2 (aq) + 2 NaOH (aq) → Pb(OH)2 (s)+ 2 NaCl (aq)
White precipitate of lead(II) hydroxide is formed.
Example: Fe2+
Fe(NO3)2 (aq) + 2 NaOH (aq) → Fe(OH)2 (s)+ 2NaNO3 (aq)
Green precipitate of iron (II) hydroxide is formed.
Reaction with aqueous ammonia
Outcome 1: No precipitate formed (Ca2+)
No precipitate is formed as there is little amount of hydroxide ions present to form precipitate with calcium hydroxide, which is partially soluble.
Note: Aqueous ammonia is a weak alkali, hence only low concentration of hydroxide ions is present.
Outcome 2: Precipitate formed (Zn2+, Al2+,Pb2+,Fe2+,Fe3+,Cu2+)
When aqueous ammonia is added to other metal cations, an insoluble hydroxide is formed.
Example: Cu2+
CuCl2 (aq) + 2 NH4OH (aq) → Cu(OH)2 (s)+ 2 NH4Cl (aq)
Light blue precipitate of copper (II) hydroxide is formed.
Example: Zn2+
Zn(NO3)2 (aq) + 2 NH4OH (aq) → Zn(OH)2 (s)+ 2 NH4NO3 (aq)
White precipitate of zinc (II) hydroxide is formed.
Lead(II) ions and aluminum ions give the same results in both aqueous sodium hydroxide and aqueous ammonia. How do we distinguish lead(II) ions from aluminium ions?
Add any solution containing chloride (or sulfate) ions. Only lead(II) ions give white precipitate with chloride ions (or sulfate ions).
Tests to identify anions
(i) carbonate (by the addition of dilute acid and subsequent use of limewater)
(ii) chloride (by reaction of an aqueous solution with nitric acid and aqueous silver nitrate)
(iii) iodide (by reaction of an aqueous solution with nitric acid and aqueous silver nitrate)
(iv) nitrate (by reduction with aluminium in aqueous sodium hydroxide to ammonia and subsequent use of moist red litmus paper)
(v) sulfate (by reaction of an aqueous solution with nitric acid and aqueous barium nitrate)
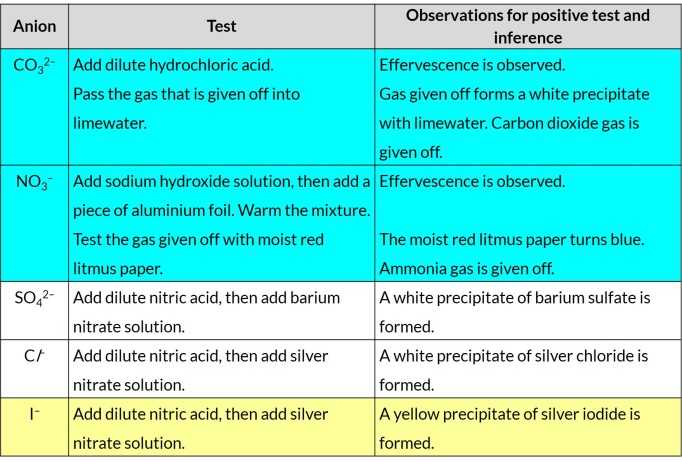
Other than barium nitrate solution, what other solution could be added to test for sulfate ions?
Hint: Recall solubility table
Lead(II) nitrate solution. White precipitate of lead(II) sulfate is formed.
Pb(NO3)2 (aq) + Na2SO4 (aq) → PbSO4 (s)+ 2 NaNO3 (aq)
Other than silver nitrate solution, what other solution could be added to test for chloride ions?
Hint: Recall solubility table
Lead(II) nitrate solution. White precipitate of lead(II) chloride is formed.
Pb(NO3)2 (aq) + 2 NaCl (aq) → PbCl2 (s)+ 2 NaNO3 (aq)
Other than silver nitrate solution, what other solution could be added to test for chloride ions?
Lead(II) iodide solution. Yellow precipitate of lead(II) iodide is formed.
Pb(NO3)2 (aq) + 2 NaI (aq) → PbI2 (s)+ 2 NaNO3 (aq)
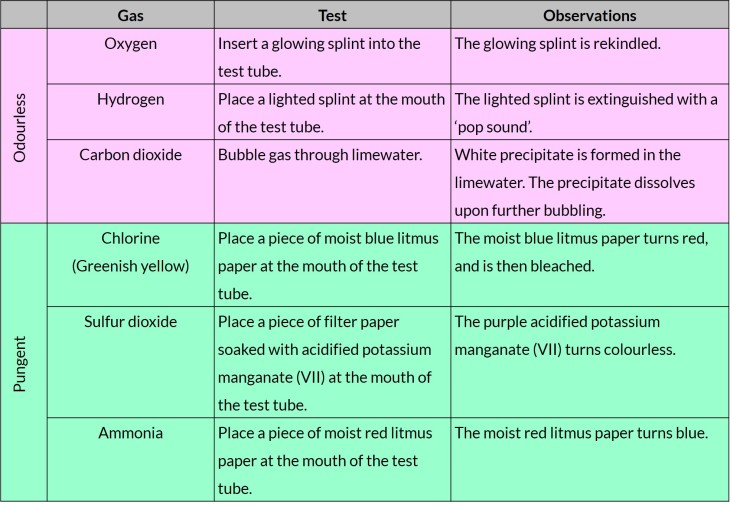
Tests to identify gases
(i) ammonia (using damp red litmus paper)
(ii) carbon dioxide (using limewater)
(iii) chlorine (using damp blue litmus paper)
(iv) hydrogen (using a burning splint)
(v) oxygen (using a glowing splint)
(vi) sulfur dioxide (using acidified potassium manganate(VII))
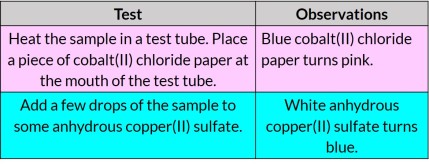
Testing for water
Describe tests to confirm the presence of water: using cobalt(II) chloride paper or using anhydrous copper(II) sulfate.