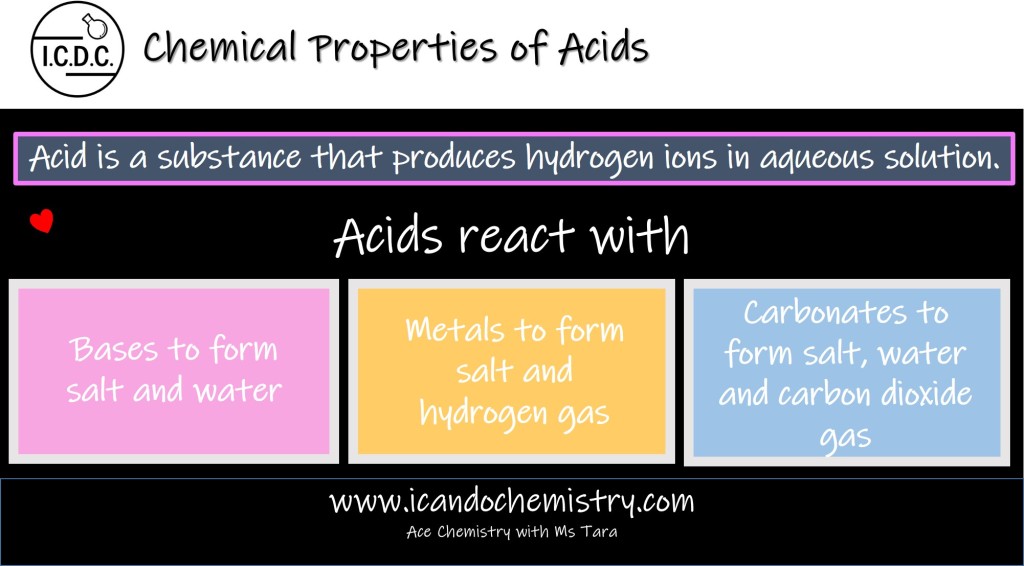
Acid is a substance that produces hydrogen ions in aqueous solution.
Common acids include Hydrochloric acid (HCl), Sulfuric acid (H2SO4) and Nitric acid (HNO3)
Acids have pH values less than 7. The lower the pH value, the more acidic the solution. Strongest acid has pH of 0.
Acids react with Metals to form salt and hydrogen gas.
Reaction between hydrochloric acid and magnesium metal:
Chemical equation: 2HCl (aq) + Mg (s) → MgCl2 (aq) + H2 (g)
Not all metals react with acids. For example, copper and silver do not react with acids.
Lead does not react with sulfuric acid and hydrochloric acid due to the insoluble layer of salt formed around the metal, protecting the metal from further reaction with acid.
Hydrogen gas can be tested with a burning splint. Hydrogen gas extinguishes burning splint with a ‘pop’ sound.
Acids react with Bases to form salt and water. This reaction is called neutralisation reaction.
Bases are either metal oxides or metal hydroxides. Soluble metal hydroxides are called alkalis.
Reaction between hydrochloric acid and sodium hydroxide is a common example of neutralisation reaction.
Chemical equation: HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Let’s work out the ionic equation of this reaction. Substances that ionises in aqueous solution are represented by (aq) state symbol. Hydrochloric acid, HCl, will ionise into hydrogen ions and chlorides ions. Sodium hydroxide, NaOH, will ionise into sodium ions and hydroxides ions. Sodium chloride, NaCl, will ionise into sodium and chloride ions. Water will not ionise, hence it is represented with (l) state symbol.
Ionic equation: H+ (aq) + Cl– (aq) + Na+ (aq) + OH– (aq) → Na+ (aq) +Cl– (aq) + H2O (l)
Any ions that appear at the left and right of the equation are considered as spectator ions. They do not take part in the reaction and can be cancelled in the equation. We will cancel Cl– (aq) and Na+ (aq), leaving us with H+ (aq) and OH– (aq).
Ionic equation of neutralisation reaction: H+ (aq) + OH– (aq) → H2O (l)
Acids react with Carbonates to form salt, water and carbon dioxide gas.
Reaction between hydrochloric acid and calcium carbonate:
Chemical equation: 2HCl (aq) + CaCO3 (s) → CaCl2 (aq) + H2O (l) + CO2 (g)
Carbon dioxide gas, an acidic gas, can be tested by bubbling the gas through limewater, Ca(OH)2. Carbon dioxide gas forms white precipitate with limewater. The white precipitate is calcium carbonate, CaCO3. CO2 (g) + Ca(OH)2 (aq) → CaCO3 (s) + H2O (l)
