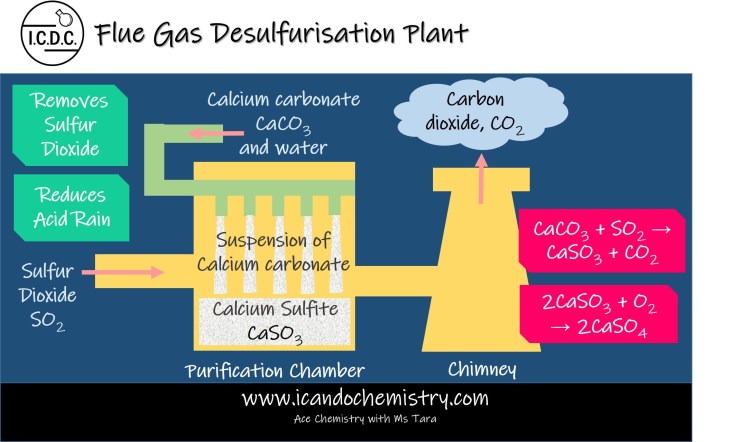
Treatment of Sulfur dioxide – Flue Gas Desulfurisation
Waste gases are produced when fossil fuels undergo combustion. Waste gases are called flue gas.
Sulfur dioxide is one of the waste gases from combustion of fossil fuels. The process of removing sulfur dioxide from flue gases is called flue gas desulfurisation.
Chemical Reactions
Sulfur dioxide gas is an acidic oxide. It is allowed to react with an wet mixture of calcium carbonate to form solid calcium sulfite. Carbon dioxide gas is also formed. Calcium sulfite then further oxidises to calcium sulfate by atmospheric oxygen, which is then dumped.
Chemical Equation:
CaCO3 (s) + SO2 (g) → CaSO3 (s) + CO2 (g)
2CaSO3 (s) + O2 (g) → 2CaSO4 (s)
Alternatively, the acidic sulfur dioxide is allowed to react with basic calcium oxide to form calcium sulfite, which is then oxidised to calcium sulfate.
Chemical Equation:
CaO (s) + SO2 (g) → CaSO3 (s)
2CaSO3 (s) + O2 (g) → 2CaSO4 (s)
