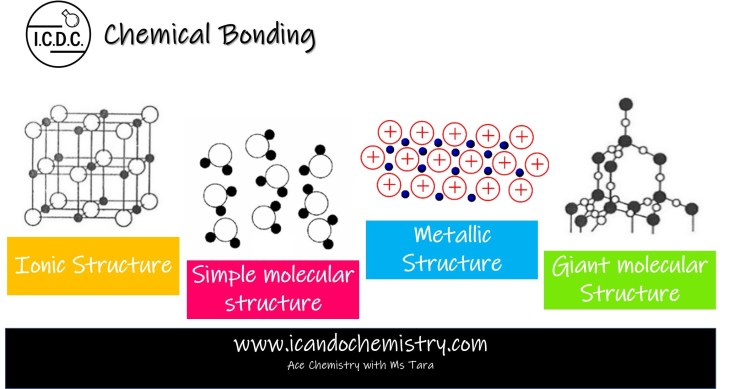
Ionic Structure
Formed between metallic atoms and non-metallic atoms by transfer of electrons. Metallic atoms form positive ions while non-metallic atoms form negative ions.
Ionic compounds form giant ionic lattice structure. Oppositely charged ions are held together by strong electrostatic forces of attraction in the giant lattice structure.
Examples: Sodium chloride, Lithium fluoride, Calcium oxide
Simple molecular structure
Formed between non-metallic atoms by sharing of electrons.
One simple molecule is made of a few atoms joined together by strong covalent bonds within molecule.
Weak intermolecular forces of attraction exist between molecules.
When a
substance with simple molecular structure is melted or boiled, heat energy
overcome weak intermolecular forces of attraction between simple molecules.
Note: The covalent bonds are not broken.
Examples: Hydrogen gas, Oxygen gas, Hydrogen chloride gas
Metallic Structure
Formed between metallic atoms where each metallic atom loses electrons to become positive ions. The delocalised electrons go into the spaces between the positive ions.
Metals have giant metallic structure consisting of a lattice of positive ions in a sea of delocalised electrons.
Examples: Metals
Giant molecular structure
Formed between non-metallic atoms by sharing of electrons. One giant molecule is made of a giant network of atoms joined together by strong covalent bonds.
Macromolecules have giant molecular structure. A giant network of atoms are covalently bonded together.
Examples: Carbon (graphite), Carbon (diamond), Silicon dioxide (sand)
