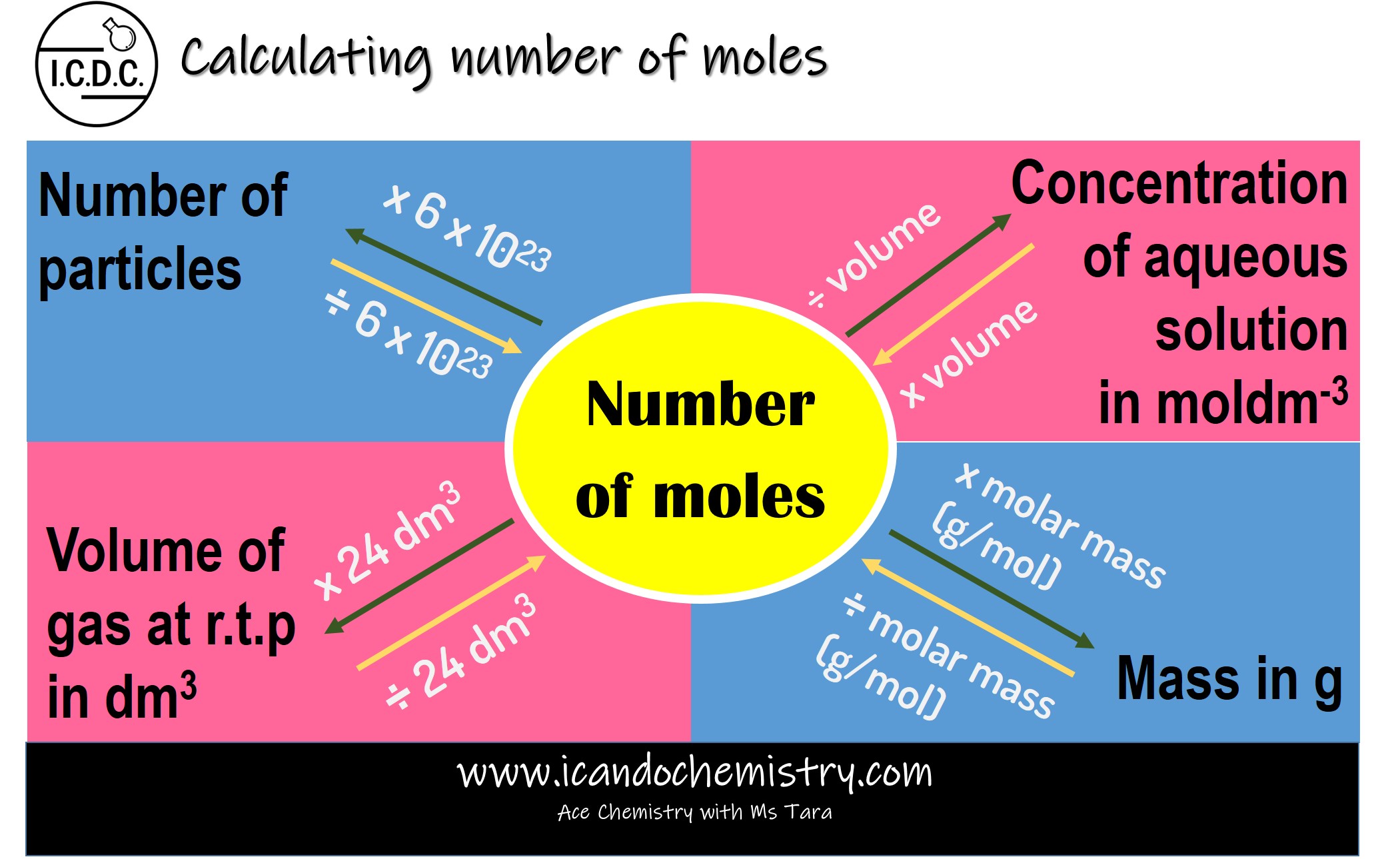
The mole is the base unit of amount of substance. One mole of substance has 6 x 1023 particles. Particles may be molecules, atoms, ions and electrons.
Number of particles
One mole of substance contains 6 x 1023 particles. This value is called Avogadro’s number.
Number of particles in 1 mol of substance = 6 x 1023
Number of particles in 2 mol of substance = 2 x 6 x 1023
Number of particles in y mol of substance = y x (6 x 1023)
Hence,
Number of particles = number of moles x (6 x 1023)
Volume of gas at r.t.p
One mole of any gas occupies 24 dm3 (24 000 cm3) at room temperature and pressure. This volume is called the molar volume of a gas.
At r.t.p,
Volume
occupied by 1 mol of gas = 24 dm3
Volume occupied by 2 mol
of gas = (2 x 24) dm3
Volume occupied by y mol
of gas = (y x 24) dm3
Hence,
Volume of gas = number of moles x 24 dm3
Mass of substance in g
Molar mass is the mass of one mole of a substance.
Take for example, copper metal
Mass of 1 mol of copper metal = 64 g (64 gmol-1)
Note: 64 gmol-1 is the molar mass of copper metal. This value is obtained from The Periodic Table
Mass of 2 mol of copper metal = (2 x 64) g
Mass of y mol of copper metal = (y x 64) g
Hence,
mass of substance = number of moles x molar mass
Concentration of aqueous solution
The concentration of a solution is the amount of solute dissolved in a unit volume of a solution.
Concentration of a solution can be expressed in gdm-3 or moldm-3.
When concentration is expressed in moldm-3, it is called the molar concentration.
Concentration of aqueous solution in gdm-3 can be calculated by multiplying concentration in moldm-3 with the molar mass of the solute
