Alkenes
Alkenes are a homologous series of unsaturated hydrocarbons with the general formula CnH2n.
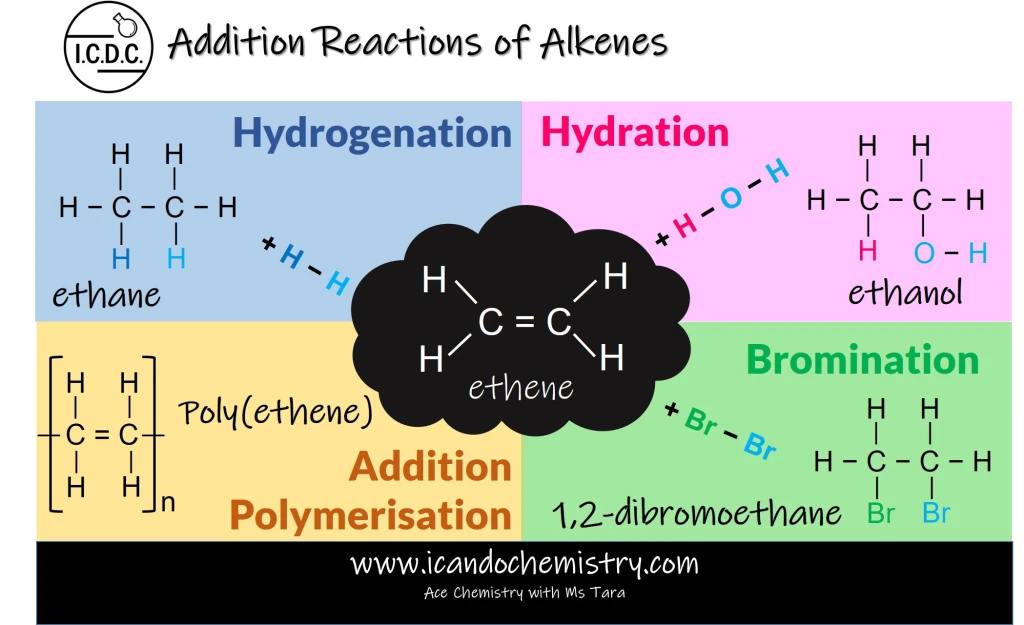
All alkenes contain the carbon-carbon double bond (C=C). This double bond is very reactive, hence, alkenes undergo addition reactions readily.
In an addition reaction, double bonds become single bonds. The unsaturated hydrocarbon becomes a saturated hydrocarbon.
Addition reactions of alkenes
Hydrogenation is the addition of hydrogen to alkenes to form alkanes.
Conditions: Temperature of 200 °C and presence of nickel catalyst.
Hydration is the addition of steam to alkenes to form alcohols.
Conditions: Temperature of 300 °C, 60 atm and presence of phosphoric (V) acid catalyst
Bromination is the addition of bromine to alkenes.
Reddish brown aqueous bromine decolourises/turns colourless in the presence of alkenes. This is a common test to determine the presence of unsaturated hydrocarbons.
Addition polymerisation is the reaction between alkenes to form addition polymer.
Conditions: High temperature and pressure, presence of catalyst
