
Ammonia is a colourless gas at room temperature and pressure.
Ammonia dissolves in water to form weak alkali.
NH3 (g) + H2O (l) ⇌ NH4+ (aq) + OH– (aq)
A weak alkali ionises partially in aqueous solution. It is the hydroxide ions formed that gives aqueous ammonia its alkaline properties.
We use ⇌ to represent partial ionisation of weak alkalis. Do not use arrows like ⇄ and ⇿.
Ammonia is manufactured industrially by the Haber Process.
Ammonia is used to make fertilisers, explosives, dyes, household cleaners and nylon.
Ammonia is an important raw material in the manufacture of nitric acid, HNO3 via Ostwald Process.
Ostwald Process is not covered in the O level Syllabus. However, it is great to know more. Hence, I will be sharing more details here.
Making of Nitric acid by the Ostwald Process
Stage 1: Ammonia is oxidised into nitric oxide and steam. This is a highly exothermic reaction.
4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (g)
Stage 2: Nitric oxide is further oxidised to form nitrogen dioxide, which is then dissolved in water to form nitric acid.
2NO (g) + O2 (g) → 2NO2 (g)
3NO2 (g) + H2O (l) → 2HNO3 (aq) + NO (g)
Nitric acid obtained is in diluted form. It can be concentrated to the required strength by fractional distillation. Nitric oxide gas is recycled.
Drawing dot and cross diagram of Ammonia
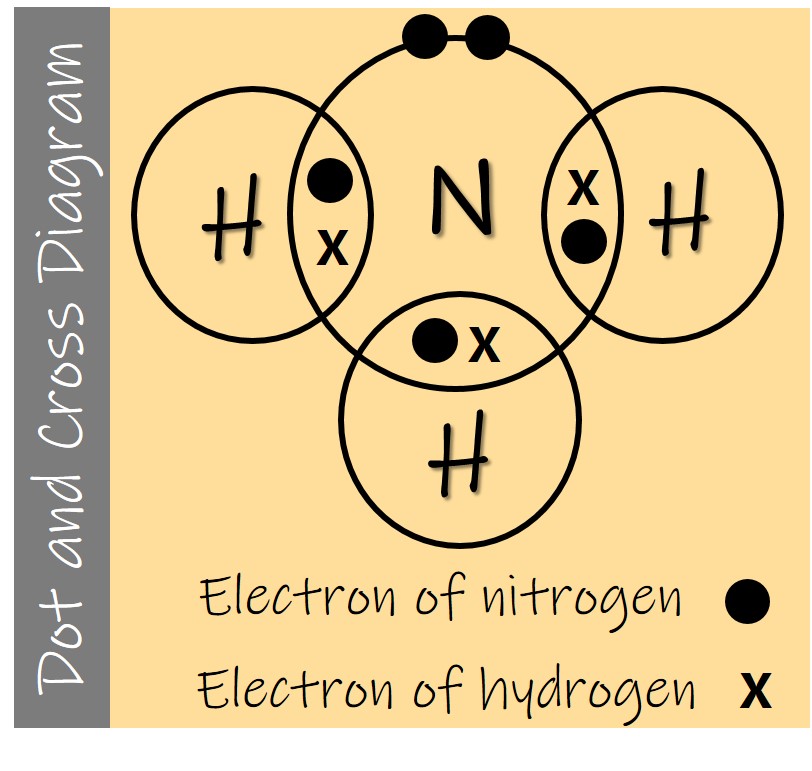
Ammonia is made of 1 nitrogen and 3 hydrogen atoms. The atoms are covalently bonded together.
Nitrogen atom has 7 electrons. Electronic configuration of nitrogen is 2, 5.
Hydrogen has 1 electron. Electronic configuration of hydrogen is 1.
Nitrogen atom shares 1 electron with each hydrogen atom. Nitrogen achieves stable noble gas octet electronic configuration of 2,8. Hydrogen achieves stable noble gas duplet electronic configuration of 2.
