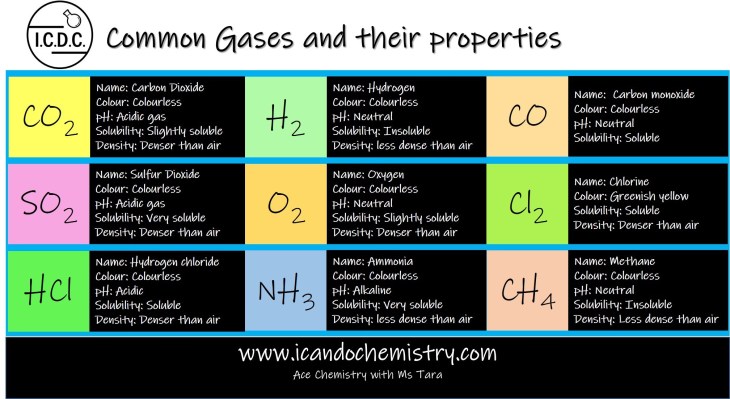
Carbon dioxide (CO2)
Carbon dioxide (CO2) is a colourless and acidic gas. It dissolves in water to form carbonic acid. H2CO3, which is a weak acid.
Molar mass of air is about 28 g/mol as there is 78% nitrogen in the air. Molar mass of carbon dioxide is 12 + 2(16) = 44 g/mol. Hence, carbon dioxide gas is denser than air.
Sulfur dioxide (SO2)
Sulfur dioxide (SO2) is a colourless and acidic gas. It dissolves in water to form sulfurous acid. H2SO3. Sulfurous acid further oxidises to form sulfuric acid, H2SO4, which is a strong acid.
Molar mass of air is about 28 g/mol as there is 78% nitrogen in the air. Molar mass of sulfur dioxide is 32 + 2(16) = 64 g/mol. Hence, sulfur dioxide gas is denser than air.
Hydrogen chloride (HCl)
Hydrogen chloride (HCl) is a colourless and acidic gas. It dissolves in water to form hydrochloric acid, which is a strong acid.
Molar mass of air is about 28 g/mol as there is 78% nitrogen in the air. Molar mass of hydrogen chloride is 1 + 35.5 = 36.5 g/mol. Hence, hydrogen chloride gas is denser than air.
Hydrogen (H2 )
Hydrogen (H2) is a colourless and neutral gas. It is highly flammable.
Molar mass of air is about 28 g/mol as there is 78% nitrogen. Molar mass of hydrogen is 1+1 = 2 g/mol. Hence, hydrogen is less dense than air.
Oxygen (O2)
Oxygen (O2) is a colourless and neutral gas. It supports combustion.
Molar mass of air is about 28 g/mol as there is 78% nitrogen. Molar mass of oxygen is 16+16 = 32 g/mol. Hence, oxygen is denser than air.
Ammonia (NH3)
Ammonia (NH3) is a colourless and alkaline gas. It dissolves in water to form aqueous ammonia. NH4OH is the alternative chemical formula of aqueous ammonia. It is a weak alkali.
Molar mass of air is about 28 g/mol as there is 78% nitrogen in the air. Molar mass of ammonia is 14 + 3(1) = 17 g/mol. Hence, ammonia is less dense than air.
Carbon monoxide (CO)
Carbon monoxide (CO) is a colourless and neutral gas. It is poisonous.
Molar mass of air is about 28 g/mol as there is 78% nitrogen. Molar mass of carbon monoxide is 12+16 = 28 g/mol. Hence, it has the relatively similar density as air.
Chlorine (Cl2)
Chlorine (Cl2) is a greenish yellow gas. It dissolves in water to form hypochlorous acid/chloric(I) acid HOCl and hydrochloric acid, HCl. In the test for chlorine gas, the moist blue litmus paper turns red, then bleaches.
Molar mass of air is about 28 g/mol as there is 78% nitrogen in the air. Molar mass of chlorine is 2(35.5) = 71 g/mol. Hence, chlorine gas is denser than air.
Methane (CH4)
Methane (CH4) is a colourless and neutral gas. It is the first member of the alkane family.
Molar mass of air is about 28 g/mol as there is 78% nitrogen. Molar mass of methane is 12+4(1) = 16 g/mol. Hence, methane is less dense than air.
