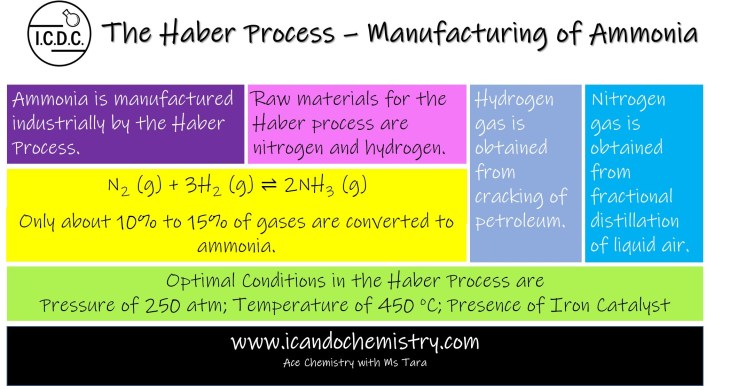
Ammonia is manufactured industrially by the Haber Process.
Raw materials for the Haber process are nitrogen and hydrogen. Nitrogen gas is obtained from fractional distillation of liquid air.
Hydrogen gas is obtained from cracking of petroleum.
Reaction between nitrogen and hydrogen is reversible.
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Reaction Conditions
To obtain the best yield, reaction conditions are meticulously controlled.
Pressure is maintained at around 250 atm, while temperature is controlled at about 450 oC.
Presence of finely divided ion catalyst is also required to speed up the reaction.
Yield of Ammonia
Only about 10% to 15% of gases are converted to ammonia.
Yield of ammonia improves with increase in pressure. However, it is dangerous to maintain very high pressure. Also, it is costly to purchase expensive equipment necessary for maintaining high pressure. In this reversible reaction, the forward reaction is an exothermic reaction, while the backward reaction is an endothermic reaction. Increase in temperature favours the backward reaction, resulting in less yield of ammonia. Decrease in temperature actually favours the production of ammonia. However, the lower the temperature, the slower the reaction. 450 oC is the optimal temperature for maximum yield at a reasonable rate.
