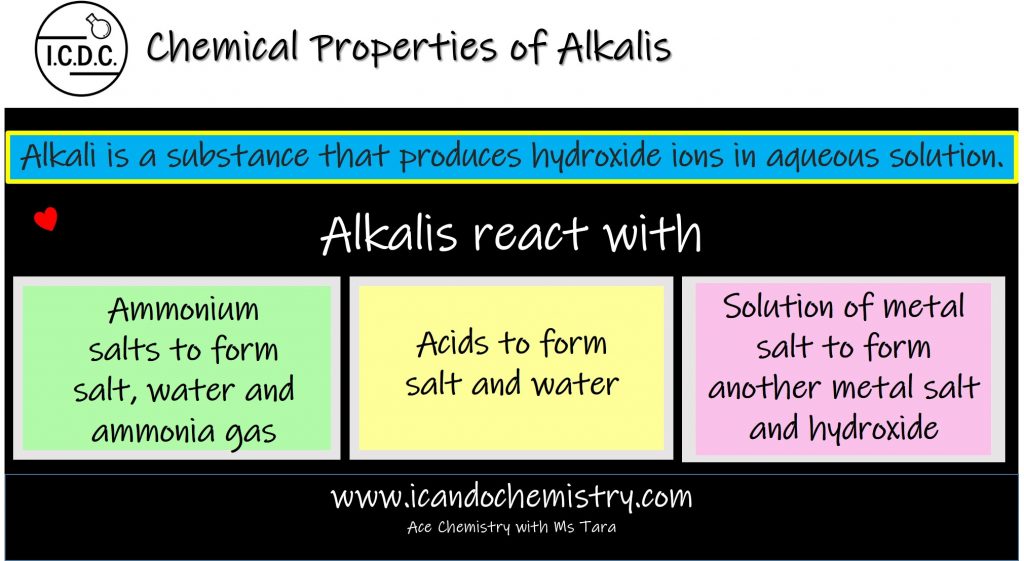
Alkali is a substance that produces hydroxide ions in aqueous solution.
Common alkalis include Group (I) metal hydroxides, calcium hydroxide and barium hydroxide.
Alkalis have pH values more than 7. The higher the pH value, the more alkali the solution. Strongest alkali has pH of 14.
Alkalis react with Acids to form salt and water. This reaction is called neutralisation reaction.
Reaction between hydrochloric acid and sodium hydroxide is a common example of neutralisation reaction.
Chemical equation: HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Let’s work out the ionic equation of this reaction. Substances that ionises in aqueous solution are represented with (aq) state symbols. Hydrochloric acid, HCl, will ionise into hydrogen ions and chlorides ions. Sodium hydroxide, NaOH, will ionise into sodium ions and hydroxides ions. Sodium chloride, NaCl, will ionise into sodium and chloride ions. Water will not ionise, hence it is represented with (l) state symbols.
Ionic equation: H+ (aq) + Cl– (aq) + Na+ (aq) + OH– (aq) → Na+ (aq) +Cl– (aq) + H2O (l)
Any ions that appear at the left and right of the equation are considered as spectator ions. They do not take part in the reaction and can be cancelled in the equation. We will cancel Cl– (aq) and Na+ (aq), leaving us with H+ (aq) and OH– (aq).
Ionic equation of neutralisation reaction: H+ (aq) + OH– (aq) → H2O (l)
Alkalis react with ammonium salts to form salt, water and ammonia gas.
For example, sodium hydroxide reacts with ammonium chloride to form sodium chloride, water and ammonia gas.
Chemical equation: NaOH (aq) + NH4Cl (s) → NaCl (aq) + H2O (l) + NH3 (g)
Ammonia gas can be tested with the use of damp red litmus paper. As ammonia gas is very soluble in water, reaction mixture is heated to drive out the gas for testing. Ammonia gas, a pungent gas, turns damp red litmus paper blue.
Alkalis react with solution of metal salt to form another metal salt and hydroxide
For example, sodium hydroxide reacts with copper(II) sulfate to form sodium sulfate and copper(II) hydroxide.
Chemical equation: 2NaOH (aq) + CuSO4 (aq) → Na2SO4 (aq) + Cu(OH)2 (s)
This reaction may be confusing at first glance. Working out the ionic equation will make things clearer.
Substances that ionises in aqueous solution are represented with (aq) state symbols. Sodium hydroxide, NaOH, will ionise into sodium ions and hydroxides ions. Copper(II) sulfate will ionise into copper(II) ions and sulfate ions. In the reaction mixture, there are four ions present, that is, sodium ions, hydroxides ions, copper(II) ions and sulfate ions. They are free to form a new compound. Copper(II) ions and hydroxide ions come together to form a solid, leaving sodium ions and sulfate ions in the aqueous solution.
As to why copper(II) and hydroxide ions form solid, recall that all bases (metal hydroxides and oxides) are insoluble except alkali, which is soluble hydroxide. Soluble hydroxides include group(I) metal hydroxides, calcium hyroxide and barium hydroxide. All the other hydroxides are insoluble, with state symbol (s).
Ionic equation: Cu2+ (aq) + SO42- (aq) + Na+ (aq) + OH– (aq) → Na+ (aq) +SO42- (aq) + Cu(OH)2 (s)
Any ions that appear at the left and right of the equation are considered as spectator ions. They do not take part in the reaction and can be cancelled in the equation. We will cancel SO42- (aq) and Na+ (aq), leaving us with Cu(OH)2 (s)
Ionic equation: Cu2+ (aq) + OH– (aq) → Cu(OH)2 (s)
