Qualitative Analysis is the process of identifying unknown substances in a solution.
Students are required to describe the use of aqueous sodium hydroxide and aqueous ammonia to identify the following aqueous cations:
– aluminium (Al3+)
– ammonium (NH4+)
– calcium (Ca2+)
– copper(II) (Cu2+)
– iron(II) (Fe2+)
– iron(III) (Fe3+)
– lead(II) (Pb2+)
– zinc (Zn2+)
The formulae of complex ions are not required.
We will focus on the use of aqueous sodium hydroxide in this video.
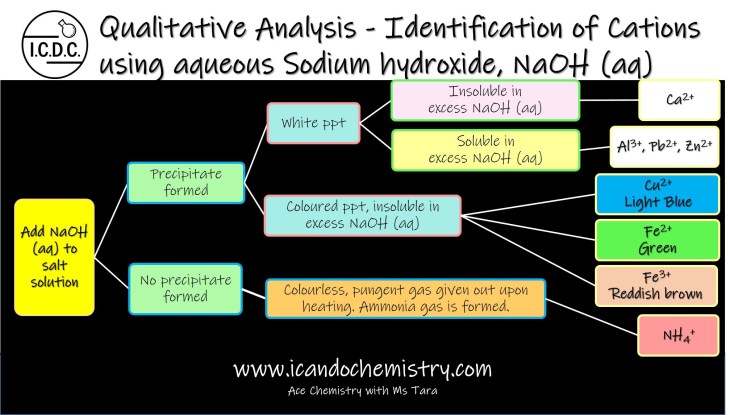
For easy memory, you may wish to first divide the cations into 2 groups – precipitate formed and no precipitate formed.
No Precipitate formed
In ‘no precipitate formed’ category, if colourless and pungent gas is given out upon heating, then we can confirm ammonium ion is present. Of course, to confirm the identity, we should test the gas using moist red litmus paper. Ammonia gas will turn moist red litmus paper blue.
Precipitate formed
Further divide the cations under ‘precipitate formed’ into white precipitate and coloured precipitate.
White Precipitate formed
Calcium, aluminium, zinc and lead(II) ions give white precipitate with a few drops of sodium hydroxide. If the precipitate dissolves in excess to form a colourless solution, then zinc ion, aluminium ion or lead(II) ions could be present. Further tests can be done to identify which of these three ions are present. If the precipitate is insoluble in excess, then calcium ion must be present.
Coloured Precipitate formed
Things get easier with coloured precipitate.
Light blue precipitate is formed if copper(II) ion is present.
Green precipitate is formed if iron(II) ion is present.
Reddish brown precipitate is formed if iron(III) ion is present.
All the precipitate are insoluble in excess sodium hydroxide.
The precipitate formed in each of the reactions is the hydroxide of the metal ion.
Click here for more examples and chemical equations on identification of cations.
Please like the video and subscribe to the channel if you find this video useful. Happy learning Chemistry!
