All matter is made of particles in constant random motion.
When a matter is heated, particles take in heat energy. Kinetic energy of particles increases, hence particles move faster.
When a matter is cooled, particles lose heat energy. Kinetic energy of particles decreases, hence particles move slower.
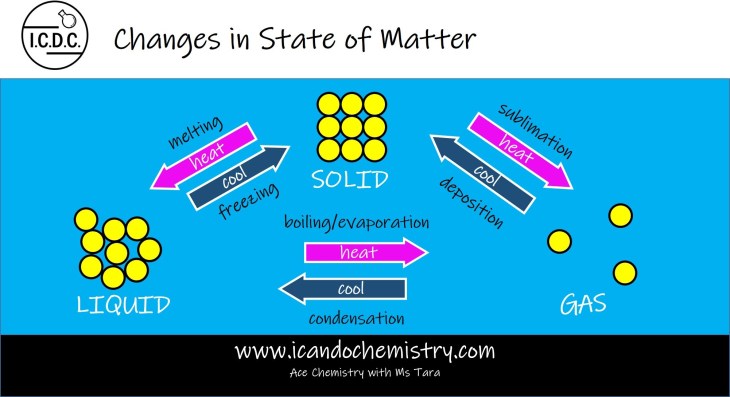
Changes in state of matter are reversible.
Melting is the process by which a substance changes from a solid to a liquid.
Freezing is the process by which a substance changes from a liquid to a solid.
Sublimation is the process by which a substance changes from a solid to a gas.
Deposition is the process by which a substance changes from a gas to a solid.
Boiling/evaporation is the process by which a substance changes from a liquid to a gas.
Condensation is the process by which a substance changes from a gas to a liquid.
