Syllabus Objectives:
Describe the use of aqueous sodium hydroxide and aqueous ammonia to identify the following aqueous cations: aluminium, ammonium, calcium, copper(II), iron(II), iron(III), lead(II) and zinc (formulae of complex ions are not required).
Cation Tests and Observations
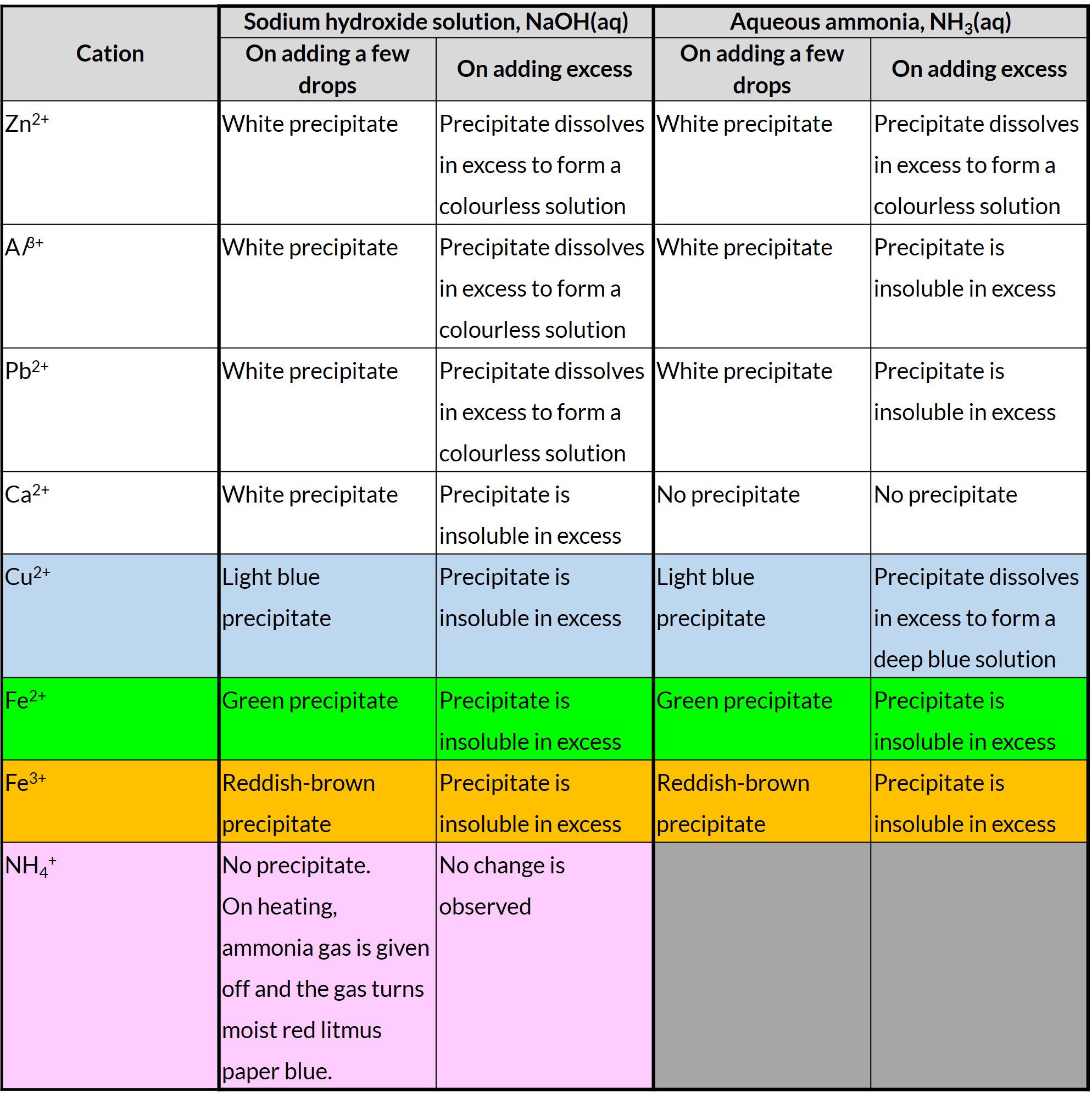
What exactly happened?
Click here for online video on identification of cations using aqueous ammonia.
Click here for flowchart on identification of cations using aqueous ammonia.
Click here for online video on identification of cations using aqueous sodium hydroxide.
Click here for flowchart on identification of cations using aqueous sodium hydroxide.
Reaction with aqueous sodium hydroxide
Outcome 1: No precipitate formed (NH4+)
Ammonium salts will react with aqueous sodium hydroxide to form a soluble salt, water and ammonia gas.
Example: Ammonium chloride + Sodium Hydroxide → Sodium Chloride (soluble) + Water + Ammonia gas
NH4Cl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) + NH3 (g)
On heating, ammonia gas is given off. Gas turns moist red litmus paper blue.
Why should the litmus paper be moist?
Ammonia, being an alkaline gas, produces hydroxide ions (OH–) in the presence of water.
H2O (l) + NH3 (g) → NH4+ (aq) + OH– (aq)
Outcome 2: Precipitate formed (Zn2+, Al2+,Pb2+,Fe2+,Fe3+,Cu2+,Ca2+)
When sodium hydroxide is added to other metal cations, an insoluble hydroxide is formed.
Example 1:
Lead(II) chloride + Sodium Hydroxide → Lead(II) Hydroxide (insoluble) + Sodium Chloride (soluble)
PbCl2 (aq) + 2 NaOH (aq) → Pb(OH)2 (s)+ 2 NaCl (aq)
White precipitate of lead(II) hydroxide is formed.
Example 2:
Iron(II) nitrate + Sodium Hydroxide → Iron(II) Hydroxide (insoluble) + Sodium Nitrate (soluble)
Fe(NO3)2 (aq) + 2 NaOH (aq) → Fe(OH)2 (s)+ 2NaNO3 (aq)
Green precipitate of iron (II) hydroxide is formed.
Reaction with aqueous ammonia
Outcome 1: No precipitate formed (Ca2+)
No precipitate is formed as there is little amount of hydroxide ions present to form precipitate with calcium hydroxide, which is partially soluble.
Note: Aqueous ammonia is a weak alkali, hence only low concentration of hydroxide ions is present.
Outcome 2: Precipitate formed (Zn2+, Al2+,Pb2+,Fe2+,Fe3+,Cu2+)
When aqueous ammonia is added to other metal cations, an insoluble hydroxide is formed.
Example: Copper(II) chloride + Aqueous Ammonia → Copper(II) Hydroxide (insoluble) + Ammonium Chloride (soluble)
CuCl2 (aq) + 2 NH4OH (aq) → Cu(OH)2 (s)+ 2 NH4Cl (aq)
Light blue precipitate of copper (II) hydroxide is formed.
Example: Zinc nitrate + Aqueous Ammonia → Zinc Hydroxide (insoluble) + Ammonium Nitrate (soluble)
Zn(NO3)2 (aq) + 2 NH4OH (aq) → Zn(OH)2 (s)+ 2 NH4NO3 (aq)
White precipitate of zinc (II) hydroxide is formed.
Question
Lead(II) ions and aluminum ions give the same results in both aqueous sodium hydroxide and aqueous ammonia. How do we distinguish lead(II) ions from aluminium ions?
Answer
Add any solution containing chloride (or sulfate) ions. Only lead(II) ions give white precipitate with chloride ions (or sulfate ions).
Click here for online video on identification of cations using aqueous ammonia.
Click here for flowchart on identification of cations using aqueous ammonia.
Click here for online video on identification of cations using aqueous sodium hydroxide.
Click here for flowchart on identification of cations using aqueous sodium hydroxide.
